Understanding Japan’s Cosmetics Labeling Regulations
Proper labeling is a critical part of introducing cosmetics and personal care products to the Japanese market. Japan’s cosmetics regulations ensure consumer safety and establish strict compliance requirements to protect both the public and brands. Misleading or inaccurate labeling can lead to costly delays, legal issues, and loss of market access. In this guide, we will walk through Japan’s cosmetics labeling regulations and provide actionable guidance for international brands looking to succeed in this market.
Japan’s Cosmetics Labeling Regulations
Japan’s cosmetics market is the fourth-largest in the world, home to around 3,000 companies, with skincare being the dominant segment. This growth is driven by rising demand for high-quality, innovative, and eco-friendly products, underscoring the importance of complying with Japan’s stringent cosmetic regulations.
The industry is governed by these key regulatory bodies and laws:
- Ministry of Health, Labor, and Welfare (MHLW):
Oversees the regulatory framework for cosmetics and personal care products. - Pharmaceutical and Medical Devices Agency (PMDA):
Works under the MHLW to review import applications and product approvals.
Japan has labeling requirements to ensure transparency and consumer safety. All labels must be in Japanese, and the outer packaging must include a complete ingredient list. These regulations are designed to protect consumers by ensuring they have accurate and clear information about the products they purchase.
Required Labeling Elements
The key labeling components required by Japanese regulations include:
Pattern 1: Inner Product Packaging Only
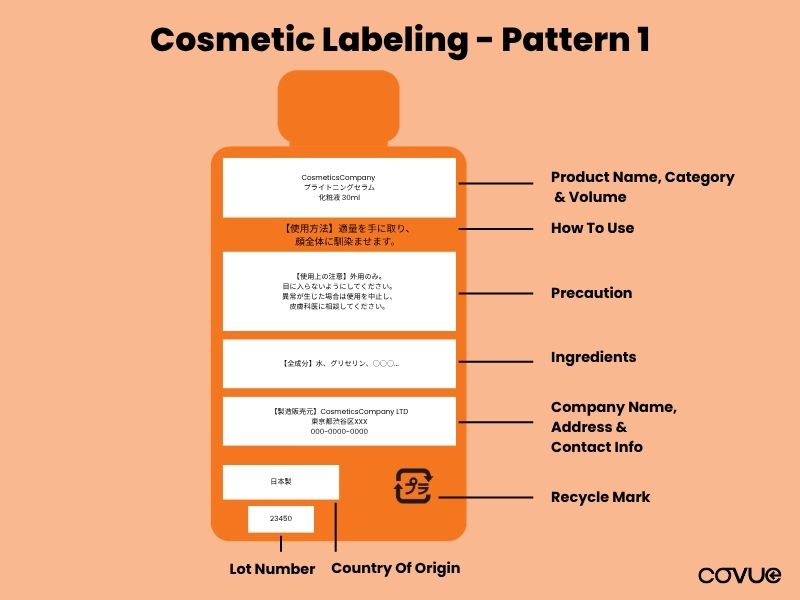
For cases where the product packaging is the entire product, and no outer packaging is included. The following items must be included on the product:
- Product Name: The official name of the product.
- Product Category: Classification, such as lotion, cream, or shampoo.
- Volume: Net content shown in millilitres (ml) or grams (g).
- User Instructions: Basic guidance for use.
- Ingredient List: All ingredients must be listed in descending order of concentration, written in Japanese, and comply with MHLW’s designated ingredient list.
- Precaution: Warnings or important safety information for storage and use.
- Company Name (Cosmetics License Holder): Legal entity responsible for the product in the market.
- Company Address (Cosmetics License Holder): Full address of license holder for traceability.
- Contact Info (Website or Phone): Optional but recommended for consumer support.
- Country of Origin: Must indicate where the product was manufactured.
- Lot Number: For product traceability in the event of a recall or issue.
- Manufacturing Date / Expiry Date: Although not mandatory, it is advised to include it on your packaging.
- Recycle Mark: Indicate the correct disposal method in line with Japan’s recycling standards.
Pattern 2: With Outer Box Packaging
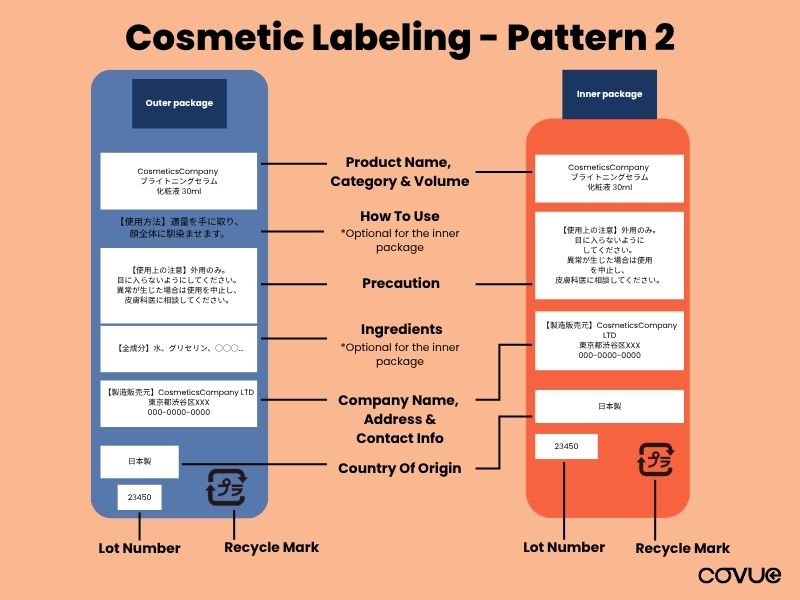
For cases where a product has both an outer and inner package (eg. bottle in a box). The following items must be included on the packaging:
| Label Item | Outer Packaging | Inner Packaging |
| Product Name | ✅ | ✅ |
| Product Category | ✅ | ✅ |
| Volume (ml/g) | ✅ | ✅ |
| User Instructions | ✅ | – |
| Ingredient List | ✅ | – |
| Precaution | ✅ | ✅ |
| Company Name (Cosmetics License Holder) | ✅ | ✅ |
| Company Address (Cosmetics License Holder) | ✅ | ✅ |
| Contact Info (Optional) | Optional | Optional |
| Country of Origin | ✅ | ✅ |
| Lot Number | ✅ | ✅ |
| Manufacturing Date / Expiry Date | Optional | Optional |
| Recycle Mark | ✅ | ✅ |
Label Formatting Requirements
Labels for cosmetics products must follow these requirements:
- Font Size: Minimum 7-point font; 4.5-point is acceptable if space is limited.
- Small Packaging Exception: Containers of 30ml or less are exempt from font size rules.
- Volume Notation: Must be clearly labeled in millilitres (ml) or grams (g).
- It is strongly recommended to use a clear, easy to read Japanese font.
Differences Compared to Other Product Categories
While cosmetics share some similarities with food, supplements, and pharmaceuticals in terms of labeling, there are notable differences:
- Cosmetics: Labels must adhere to strict regulations, especially concerning safety and efficacy claims. Therapeutic claims are generally prohibited unless the product is classified as a quasi-drug.
- Food and Supplements: Food products must include nutritional information and allergens, and supplements can make health-related claims if they comply with the health claim regulations. These categories also require ingredient disclosure but focus more on nutritional content.
- Pharmaceuticals: Pharmaceuticals can make specific therapeutic claims, but they must be approved by MHLW and PMDA. Labels must also provide dosage instructions and medical warnings.
The key distinction is that cosmetics are not allowed to make therapeutic or medicinal claims unless classified as quasi-drugs. Additionally, for quasi-drugs that make claims, approval from the Ministry of Health, Labor and Welfare (MHLW) is required based on the active ingredients. MHLW recognizes the product’s effectiveness, and as a result, these products are often referred to as medicated cosmetics.
Common Labeling Compliance Challenges
Many international brands encounter challenges when complying with Japan’s cosmetic labeling regulations:
- Misleading Claims: Making unapproved claims about a product’s safety or efficacy can lead to rejections or fines.
- Ingredient Listing Errors: Failing to list ingredients in the correct order or missing critical ingredients can lead to delays.
- Labeling Language Issues: Since Japanese is mandatory, incorrect or incomplete translations can cause compliance issues.
- Incorrect Claims: Claims like “recommended by doctors” or “clinically proven” are prohibited unless approved by MHLW.
Non-compliance can result in import delays, fines, or even market withdrawal.
How to Ensure Compliance
To ensure smooth entry into Japan’s market and compliance with cosmetics labeling regulations, brands should consider these best practices:
- Consult Experts: Work with regulatory experts who understand Japan’s laws and requirements.
- Use Professional Translators: Ensure your product labels are accurately translated into Japanese to avoid confusion or errors.
- Conduct a Pre-Market Check: A Pre-Market Check can help ensure your product and label comply with all necessary regulations before submitting documentation for approval.
- Stay Updated: Regulations can evolve, so staying informed about any changes to Japan’s labeling requirements is crucial for ongoing compliance.
How COVUE Can Support You
Proper labeling is not only a legal requirement in Japan, but it also builds trust with consumers and ensures a smooth market entry. By following Japan’s stringent cosmetic labeling regulations, brands can avoid costly mistakes and position themselves for success. If you’re planning to expand into Japan, COVUE is here to help with regulatory support and labeling compliance to ensure your product meets Japan’s high standards.
Contact us today for expert assistance with your Japan market entry and labeling needs.


